If you completed the Anatomy of an Eggshell lab you will already know that eggshells are made from Calcium Carbonate and that directly underneath there is a very strong, yet flexible, membrane. In this lab, The Embarrassed Egg and Osmosis experiment, we will dissolve the egg shell until the egg is naked and wearing only it’s skin, the membrane. Then, using the embarrassed egg we will explore the concept of osmosis and the similarities to human cells.
Lab Supplies for the Embarrassed Egg Experiment
- 3 Eggs
- 3 Jars
- White Vinegar
- Corn Syrup
- Wax Paper
- Printed lab sheet for egg experiments.
Lab Sheet
Directions to Undress your Egg

Gently position each raw egg in a jar and add white vinegar. The eggs should be fully submersed in vinegar. If the eggs float, find something to hold it down. We found a spoon was sufficient to keep the eggs submersed.
Since the eggshell is 95% calcium carbonate and the rest is made of calcium, magnesium, and proteins, small bubbles will begin to form on the eggshells almost immediately. This is because the vinegar contains acetic acid which chemically reacts with the calcium carbonate. During the reaction, water and vinegar evaporates and carbon dioxide is released into the air.

Let the eggs bask for 24 hours.
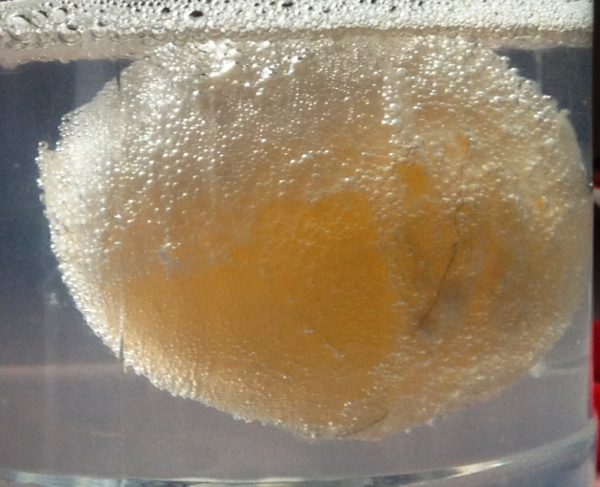
Time to play with one of the Eggs
(24 hours later)
For now, ignore two of the eggs which will remain soaking in the vinegar. Choose one egg, remove it, and gently wash it off.
In 24 hours, the egg shell has mostly dissolved. You might have to rinse and rub some of the calcium carbonate from the egg. How does it feel? The word ‘egg’ is a noun (an egg is a ‘thing’) and adjectives describe a noun. How many adjectives can you think of that describes the egg? Does it feel squishy, soft, spongy, maybe bouncy? You can probably think of more!
Place a large, flat plate in the bottom of the sink, or if you don’t mind a mess, just place a piece of wax paper on the counter top. We played with our egg on the counter top, carefully, and then resorted to using the sink when we tried dropping the egg from a higher distance.
What-if?
What-if you measured how high you can make the egg bounce? How many inches away from the counter can you bounce the egg without it breaking? Record your measurements and let us know what happened in your lab!
When the egg breaks, help clean it up, and wash your hands! Luckily, we still have two eggs left!
Embarrassed Eggs 2 and 3
When you are finished playing with the bouncy egg, it is time to pay attention to the other eggs.
Directions:
- Pour the remaining, foamy vinegar from jars 2 and 3 down the drain.
- Refill jar 1 with fresh vinegar.
- Mark this jar, using tape, with the word ‘control‘.
- Gently rinse the eggs.
- Replace one egg in the fresh vinegar. (This is your *control egg)
- Place the other egg in the container and cover it with corn syrup.
Just to review, we now have two eggs left: the control egg in vinegar, and the second egg in corn syrup. These eggs will sit in the vinegar and corn syrup for another 24 hours. Don’t disturb either because the results will be interesting!
What do you think will happen to the egg in the corn syrup?

Why Do We Need a Control Egg?
We are going to be creating a change in one egg several times, therefore we need to have ‘one’ egg that remains constant. This egg will not change so it is called our ‘control egg‘. When the second egg is in a ‘state of change‘, we can refer to the control egg as a sample of what the changed egg use to look like.
24 Hours later
Remove the eggs from the vinegar and from the corn syrup. Is there a difference? Be careful with the egg in corn syrup, we are going to use it in another lab!
How would you describe the egg that was in the corn syrup?
The Control Egg
The control egg in vinegar is still naked and has not changed, which is exactly what the control egg is suppose to do – not change. It is still swollen and larger than when it was an egg with a shell. If you are not sure, get a raw egg and set the side by side. You will see that the raw egg is smaller in comparison.
Osmosis Lab Sheet
The following lab sheet will aid with the explanation of osmosis and the hypertonic and hypotonic environments in the next lab.
Change by Osmosis
In this example, the vinegar had more water molecules than the egg itself. The water molecules, in effort to equal out the water on each side of the membrane moved from the vinegar, through the membrane, to the egg. Thus the egg swelled.

The control egg shows just how much the other egg changed. The egg removed from corn syrup looks like a very deflated, flat tire. It lost mass because the water molecules passed through the membrane to the corn syrup solution. The molecules try to equal out the environments on each side of the membrane. When one side has a higher concentration of water, the water molecules moves to the side with less water concentration. The swollen egg had lots of water molecules but the corn syrup didn’t because it had more sugar molecules.
Every time water molecules pass back and forth through the membrane it is called Osmosis.

Osmosis Explained
Think about what we learned in “Anatomy of an Egg Shell” and in “The Development of a Chick“. Water and gases can move through (permeate) the membrane of the egg. This helps the chick get oxygen and water. It also allows carbon dioxide to escape. Again, the movement of water through the membrane is called osmosis.
Small molecules can move through the membrane but large molecules cannot. The membrane is semi-permeable, meaning healthy stuff like gases (oxygen) and water can pass through, but unwanted molecules that do not create a healthy environment are not able to penetrate the egg. Unwanted molecules might mean bad news for a growing chick.
So What is the Big Deal?
It is a big deal – for a chick and for humans! We have lots of cells inside our body and they allow some substances to pass through the cell wall while other substances are blocked. For the normal person, it is difficult to create a lab where we can play with human cells and create osmosis. Instead, we can use an egg, in place of a cell, to understand why and how osmosis occurs.
BUT whatever shall we do with this sad, deflated egg?
Let’s Transfer the Water Back!
We have already used vinegar to dissolve the shell and we know that water molecules from the vinegar will transfer through the egg membrane. Let’s try something different. Will regular tap water work?
Directions:
- Put the control egg back in the vinegar.
- Wash the corn syrup molecules out of the other container.
- Add regular tap water to the container.
- Add the deflated egg.
- Wait 12-24 hours.
12 – 24 hours later…
What does the once deflated egg look like? How does it compare to the control egg?
Can you see why we have a control egg? It allows us a method of comparison each time we create a change in the other egg specimen. The once deflated, sad egg is now swollen and hydrated again!

*PARENT NOTE*
This is a lab meant for elementary students. While, it is true that osmosis is the movement of small molecules passing through a membrane, it is a bit more complicated than just movement. The rest of this lab will discuss hypertonic and hypotonic environments. For early elementary students, it might be sufficient to visually understand that vinegar made the egg swell and corn syrup caused deflation.
If you want, you could continue the lab using different solutions. Once the egg re-hydrates, what happens when you put it in another solution – like coke, colored water, or orange juice? Continuing to ask questions will instill the fact that osmosis is the movement of water and that different solutions will magically cause movement of water molecules which in turn, affects the egg.
The Rest of the Osmosis Story
The movement of water, to or from the egg, occurs when the areas on each side of the membrane have a difference in water concentration. We already know that osmosis means that water will move from an area of high water concentration, through the membrane, to the area of low concentration of water. Let’s talk about the scientific names for each of these environments.
Hypertonic vs Hypotonic Environments
When the naked, embarrassed egg, was placed in vinegar, there was a lower concentration of water inside the egg and a higher concentration in the vinegar. Since the high concentration of water is on the outside of the egg, water molecules from the vinegar moved through the membrane into the egg. This is called a “hypotonic” environment, because the water from the vinegar moved into or entered the egg, making the egg swell.
A good way to remember Hyp-O-tonic is to consider the ‘o’ in hypo. The water transferred into the egg making it swell into a large ‘O’. Another way to remember this is to think of the swollen egg as a cute, hippO – as in HIPPOtonic! Please, just don’t forget that the real word is hypotonic.

Hypertonic Environment
The corn syrup had an opposite affect on the egg when it was placed in corn syrup. The high concentration of water was in the egg. In an effort to equalize the water on each side of the membrane, water left the egg and passed through the membrane to the corn syrup, and the egg lost mass.

When water leaves the egg or cell, it is called a hypertonic environment.
One way to remember this statement is to think about “hyper” kids, I mean molecules. They want to go out to play! When hyper kids (molecules) go out and play, they burn calories, and loose weight or shrink. When the water left our egg to go to the corn syrup, the egg shrunk.
Recap:
Hypotonic environment: Water molecules pass through the membrane and ENTER the egg or cell. The egg or cell swells.
Hypertonic environment: Water LEAVES the cell through the membrane. The cell shrinks.
By dissolving the egg shell, we allowed water from the vinegar to pass through the membrane into the egg. (Hypotonic) By inserting the egg into corn syrup we extracted (withdrew) the water from the egg to the corn syrup. (Hypertonic) Then by taking the sad, deflated egg and reinserting it into regular tap water or vinegar, the water transferred back into the egg through the membrane. (Hypotonic)
What-if
What-if you tried other substances? Milk? Juice? Salt water? How would the embarrassed, naked egg react?
What if you tried colored water? Would the egg change colors?
The Embarrassed Egg and Osmosis Notebook Page
The following notebook page can be printed if you are keeping a science lab binder. It also offers an answer guide for The Embarrassed Egg and Osmosis Student Lab Sheet.
Note:
This is an introductory explanation to osmosis. It is meant solely for elementary and Jr. High students. To be able to speak to both ages, I left out terms like solvent (water) and solute (dissolved solvent). Adding these term will be in a future post. For this age level, I felt that discussing the term water and concentration was sufficient to teach the general idea of osmosis.
New to Moving Mountains Daily? Subscribe and receive lists of our favorite living books plus our monthly newsletter so you can stay up on the latest!


