Every kid loves rainbows and adults love genuine looks of surprise. The following Colorful Chemistry Lab will accomplish both! It is an easy lab for kids of all ages. When I introduced this experiment in my science classes, the students were genuinely excited to watch the cabbage juice indicator change colors and then sort the acids from the bases.

Colorful Chemistry Goals
Our goal is to use cabbage juice and household products to create a liquid rainbow of colors – and – a lot of ‘oooo’ and ‘ahhhhs’. In addition, older students will learn to determine if a substance is considered an acid or a base using a standard pH scale.
Acids and Bases
All substances can be separated into two groups: acids or bases. Acids break things down and is also known as corrosion. That’s why vinegar (an acid) makes dirty faucets and windows gleam. It works to break down grimy dirt. On the other hand, baking soda is a base and causes bubbles that makes desserts light and fluffy.
By matching the color on our rainbow scale even the youngest will have fun determining the differences. Our lab will also answer why foods taste different and why we prefer some foods more than others. Why take an antacid for an upset stomach or that nasty pink stuff. Why do some products feel slippery while others are sticky?
We can answer all these questions just by boiling cabbage!
Colorful Chemistry Lab Supplies

- Small Head of Red Cabbage
- Knife
- Pan
- Water
- Stove
- Strainer
- A container to keep the strained juice
- Several dozen small clear cups (Medicine cups, plastic cups, communion cups)
- Products for testing.
- Free Printable Lab Sheet
Safety Notes
This is a family lab and adult supervision should always be present! Adults will need to do the chopping, boiling, straining, and making sure that the cabbage liquid cools before children begin the lab.
Note: Children should never be allowed to use bleach or ammonia on their own. Always mix one substance at a time, because mixing the wrong substances together can cause dangerous chemical reactions.
Products for Testing

You can test any type of product in your household. Here are some suggestions: vinegar, lemon juice, hand sanitizer, soapy water, water, club soda, Coca Cola, orange juice, laundry detergent, tea, or milk. Non-liquid items, like sugar, salt, baking soda, baking powder, and toothpaste can be mixed with water for testing. The list is endless!
I always hesitate to tell kids what products will produce an exact rainbow because I want them to have fun investigating and exploring the possibilities. However, if you want to prepare ahead of time, we created a ‘cheat sheet’ of product testing suggestions.
*We had a difficult time with yellow. Turmeric produced the best yellow color.
Cabbage = A Chemical Indicator
A chemical indicator will change color when it reacts with s substance and the color change will show whether it is an acid or a base. Red cabbage juice is a natural indicator and is safe for children to use. However, you can also use litmus paper. These small strips of indicator paper can be purchased or you can soak coffee strainers with cabbage juice. When the strainers are dry, cut them into strips. Litmus papers change color when dipped into a liquid. From personal experience, my students have always preferred the cabbage juice indicator method!
Getting Started

To get started, slice a half head of red cabbage, add it to a pot of hot water, and simmer it for about 30 minutes. Strain the liquid into a container, by using a kitchen colander, and set it aside to cool. It is the liquid that you will be using in this lab, so don’t drain it into the sink! While the cabbage juice is cooling, gather the products you think you would like to test.
I know that some of you are sticklers for following exact recipes. Some of us fall into the other category and I happen to be one of those. Take a deep breath and put some cabbage into a soup pot – you can’t go wrong. Use the left over cabbage and make slaw!

Some teachers use a blender to make pulp and then use cheesecloth to strain the cabbage. There is no need. Keep it simple and use a kitchen strainer to separate the cabbage pulp from the juice.

Lab and Observation Time!
Kids: This is the fun part! Assemble some household products listed above and feel free to choose some of your own ideas or use my list. Make sure an adult helps you with strong cleaning solutions!
Fill a cup half way with the cabbage juice then add a household product. Observe what happens!

Lab Challange
The purple cabbage liquid changes color instantly dependent upon the substance you choose! Your lab challenge is to create a rainbow. Keep track of your results on the Colorful Chemistry Lab Sheet by matching the color to the rainbow ruler and writing down the name of the product.
That is one smart cabbage head!
Cabbage juice is referred to as a pH indicator, meaning it can determine the amount of acid or (base) alkalinity in a product. How crazy is it to call someone a ‘cabbage head’ if they do something dumb, because, in reality, a cabbage head is very smart. It is an indicator that cannot be fooled!

What makes Cabbage Juice Change?
Cabbage juice contains an indicator pigment called an anthocyanin. A very acidic liquid, like vinegar will turn the anthocyanin pigment molecules to an orange color. If there is a low alkalinity (base), then the color will turn shades of blue and purple. A good way to remember the separation of acids and bases is to think: ‘B’. Bases turn Blue. The Acids are at the red end of the scale
Anthocyanins
Anathocyanin is responsible for the red, purple, blue and white coloring in our foods. Strawberries, blackberries, blueberries, egg plant, and cauliflower are healthy foods that contain anthocyanin. Many dietitians will tell you to make your dinner plates colorful because this is one healthy enzyme!

What does pH stand for?
pH either stands for the ‘power of hydrogen‘ or the ‘potential for hydrogen – dependent on who you talk to. The most important part is that the ‘H’ stands for hydrogen.
Acids or Bases
A hydrogen ion has a tiny electrical charge. It is sorta like rubbing your feet on the carpet and then sending a little ‘zing’ over to your unsuspecting sister. If a substance has a lot of hydrogen ions, it has a lot of ‘electrical charged zing’ and will test as an acid.
If the substance has hydroxide ions then it is a base. Hydroxide ions also have an electrical charge but the molecule is different. Bases are the opposite of an acid and are sometimes referred to as alkaline.
In some instances, it is easy to determine an acid from a base. Acids feel sticky (sugar) and some taste sour (lemons) while bases, are the exact opposite. They feel slippery or slimy (baking soda) and taste bitter (coffee). BUT what about substances that we cannot taste? We certainly wouldn’t want to taste laundry detergent or lick a bar of soap! Scientists will use a chemical indicator and they will determine where the substance falls on the pH scale. Just like we did in our lab!
What is a pH scale?
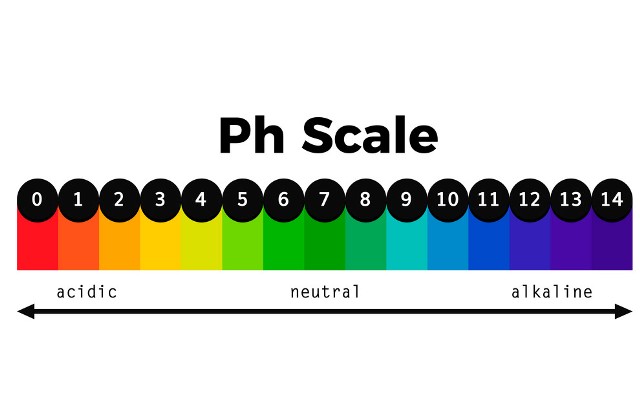
The pH scale, like the one on your lab sheet, is a type of ruler that scientists use to measure whether a substance is an acid or a base.
The pH scale ranges from 0 – 14. Acids range from 0-6 dependent on weakness or strength of the acidity. Pure water is neutral, a number 7, on the scale. Bases are opposite of an acid and range from 8 – 14, again, the color or point will depend upon the weakness or strength of the base.

Acids or Bases – Big Deal!
Actually, it is a big deal because these two groups react with each other and affect our daily lives, as well as our body functions. The types of food we eat can increase or decrease the acid levels in our bodies. These foods are broken down by strong stomach acids and when the digested food empties out of the stomach, it leaves as a base or acid compound. That’s when the kidney’s take over. They have the important job of balancing the pH level by adjusting or removing the right amount of acids. Our working organs are so amazing!
More on Acids and Bases
This is really disgusting, but when we have the flu and throw up, you can immediately feel the burning sensation and sourness from the acid in your stomach. If you could test stomach acid it would be about a 2.5 on the pH scale. *I suggest you skip this test!*
On the other hand, when you are sick, Mom might give you an antacid tablet to settle your acid-y stomach because an antacid tablet is a base. Since bases are opposite of acids, the antacid tablet will neutralize or lower the acidic pH level in your stomach. Take care of the acidity and the issue goes away!

Watch out for Fresh Pineapple
Orange juice, lemon juice, and fresh pineapple are acidic they are about a 2.5 – 3 on the pH scale. Straight lemon juice will make your face pucker because it is sour and fresh pineapple tastes sweet, but easily irritates the skin. When you eat fresh pineapple, the acidity of bromelain (an anti-inflammatory enzyme) eats away at the soft tissues in your mouth. To prevent irritation and canker sores that bromelain can cause, you need to rinse your mouth with water directly after eating pineapple. However, you will also notice that water tastes amazingly flat. This sensation is because of the neutrality of the water against a sweet tasting acid.
What-if Questions
Now, that you understand more about acids and bases, you can take it further by asking what-if questions. Here is a one to get you started.
Test the pH balance of your own spit (Ewww!) and mark it on the scale. If you swish, drink, eat, or brush with a substance will the pH balance change? What-if you chew gum? What-if you brush your teeth? What if you use fluoride vs non-fluoride toothpaste? Let us know what you come up with!
What are your what-if questions?

What-if Extra Lab
To find out more about acidic or alkaline foods, do some research on your favorite foods then create a collage separating them into acids and base categories. You might be surprised at what you are eating!
Underneath your collage, draw a kidney. Would it be a happy kidney or an overworked one?
Send us a photo of your findings and permission to use your collage. We will choose a few to post!
Fact
When you eat a lot of acidic foods, drinking neutral water can help neutralize the sugar in your blood stream! SO, drink water!!!!

no two zebras have the same stripe pattern?
Have you signed up for our monthly newsletter? We will keep you updated on our new posts as well as all the ‘latest’!


